Radioactivity and radiation
If you check Wikipedia or any chemistry course, you have some idea of what is an atom, what is radiation, what is radioactivity, and so on. Why is radiation dangerous? Because it can break the DNA in our body and that makes you sick. How much does it can break depends on the radiation energy, on how much energy our body absorbed and so on. In a way, it depends on how strong the offense is and how strong the defense is. To know more about this, first we should know about the concept of radioactivity, radiation, and their strength. Since we don’t know the meaning of those things, we are left with trusts the authorities on these matters. I would like to avoid such situation. Here I summarize the meaning of radioactivity, radiation, Becquerel, Sievert, and so forth.
We can use an analogy with light to think about these substances.

Radioactivity is also known as radioactive decay or nuclear decay. It is the process of a nucleus of an unstable atom losing energy by emitting radiation to move to a more stable state. If an atom can emit radiation, it is considered radioactive. From an observer point of view, saying that something is radioactive means that a substance can emit radiation. It is an ability of emitting radiation. If you think about a model with an offense and a defense, this is the offense side. If you think about this as light, a light bulb has the ability to emit light, so I could say that a light bulb is light-active. In this analogy, the emitted light is itself radiation. Figure 6 shows how a light bulb and a radioactive emitter that correspond to each other. Radioactivity is more like a property of a substance, that is similar to the property of a light bulb. Please note, radioactivity does not correspond to emitted light, but rather to a light bulb. A light bulb has the ability to emit light, but a light but is not light per se. We can grab a light bulb and move it to a box, then we can take it out from the box, but we cannot grab light and put it in a box. We can also use the analogy of sound. A speaker has the ability to create sound, so we could say it is sound-active, but, the speaker itself is not sound. I hope by now you know the difference between radioactivity and radiation.
Radiation is the emission of energy from the nucleus. When a nucleus changes the state, an emission happens. What kind of radiation will be emitted depends on the type of nucleus. The unit this quantifies how many times the decay happens in a second per a certain unit mass is called Becquerel (Bq). This is the strength of the offense side as we were saying earlier. A certain unit mass means for example 1kg or 1t, it is just some amount of mass.
Why do we need a certain unit of mass? Because even if we only have the same Bq radioactive substance, when we have more of it, the number of decay events increases. It is same as saying that if we have more light bulbs switched on, we get more light. Therefore, usually we see this amount of number of decay per kg (Bq/kg). If 1kg of radioactive waste is 8000 Bq it means that this 1kg of radioactive waste has 8000 decays per second. If there is 2kg of this waste, you observe 16000 decays per second. When the number of decays per second (Bq) increases, the corresponding radiation energy will also be increased. However the Bq/kg in this example is the same in both cases, that is 8000 Bq/kg. If an article only said Bq without ‘/kg’, then that should be the number of decay per second. If the certain unit of mass is smaller, then the number becomes smaller even if radioactivity stays the same. 8000Bq/kg is equal to 8Bq/g. If some food has 1000 cal/kg, it is the same as 1 cal/g. Please be careful with units.
Sometimes a criterion is defined on Bq/kg. For instance, a safety criterion defines that water is safe if it is less than 10Bq/kg. Personally, I have a problem with this criterion. Since you can add non-contaminated substances to mix with contaminated waste, you can fulfill this criterion. For example, if you have a 1kg 8000Bq/kg waste, if you mix it with a clean 1kg substance you have 2kg 4000Bq/kg waste. If you have high Bq/kg tritium water, you can just put more water and achieve low enough waste water that allows to throw it in the sea according to this criterion. If any poisonous substance has this density criterion (which sometimes make sense), we could put it in the sea and pass this safety criterion. Do you think we can continue with that? I find this is dangerous. For me this density criterion (Bq/kg) is questionable. At least I would like to know how much is the absolute value, like some “8000Bq/kg waste 10kg was trashed.” If you understand this unit (Bq/kg), I think you know what kind of information you would like to know more. If I only have the Bq/kg, I see that the information — How much waste have you actually trashed? — is hidden away.
Amount of radiation (energy)
By now, you see that the real danger is radiation rather than radioactivity. We cannot switch off radioactive substances in order not to emit radiation. (On the other hand, we can switch off a light bulb in order not to emit light.) Thus, radioactive substances are of course dangerous. But, we could block or weaken the radiation from radioactive waste if we can put it in a thick container of lead. Therefore, if radioactive waste is well under control, we can stop the radiation. Then there is no harm to life. The problem is not like in a situation, where some radioactive substance is dispersed in the environment. In that case, how much radiation is emitted is important.
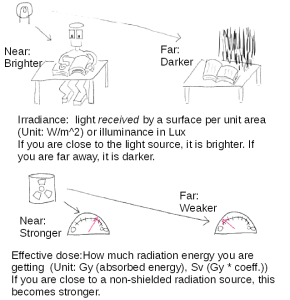
A dosage shows how much radiation is there. To say that more exactly, a dosage shows how much radiation is absorbed by something. But we could think about this as first how much radiation is there and then how much that affect a person. Figure 7 shows an analogy between light and radiation. If you go away from the light source, the brightness your receive (irradiance) becomes lower, which means it gets darker. In that Figure you see two kind of units. For radiation, we consider the energy absorption instead of radiation energy itself. You see two kind of units, Gy (gray) and Sv (Sievert). Why do we consider the energy absorption? Because if a human body is exposed to the same radiation energy, the effect also depends on how much radiation energy was absorbed by the body. For a measurement about safety, the amount of absorption is important. We measure how much energy is absorbed by gray (Gy). It is defined as the absorption of 1J of radiation energy per 1kg of matter. (If you don’t know about what is 1J, Please look up what it is.) You can imagine that thinking of the amount of energy absorption is similar to thinking of how much damage you got.
If you play some role playing video games, you know the damage depends on what is your armor or how you got the damage. That is represented by decreased your health points. In these games, what is important is how many health points you lost, not how strong the attacker is. The amount of absorption for radiation is like the amount of decreased the health points. But actually the most important value is the remaining health points, not their past decrements. However, the remaining health points for radiation depend on the person, age, and so forth. There is no simple criterion for safety, but the amount of absorbed energy could be one of such criteria.